Lithium Ion Conducting Solid Electrolyte Lanthanum Lithium Titanate (LLTO)
(Toho Titanium Co., Ltd.)
Summary
The LLTO crystal has a perovskite structure and is chemically very stable. In the future, it is expected to be applied to multi-layer solid state batteries and large cells for EVs. Low-temperature sintering and reduced resistance are key points in realizing co-firing with cathode and anode active materials as shown in the figure below. Toho Titanium is developing ways to meet these challenges.
Rechargeable Solid-State Battery
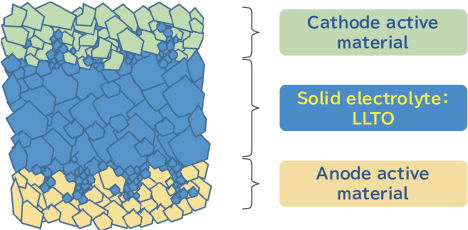
Features of Newly Developed LLTO Products ( La0.57Li0.29TiO3)
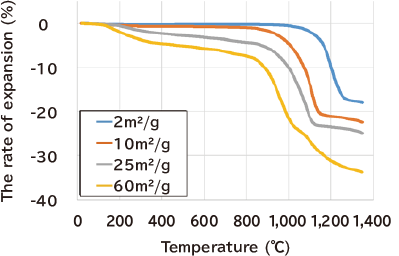
- Realization of low-temperature sintering by nanoparticle formation: Sintering temperature is reduced by nano-particle. formation (60m2/g) .(TMA)
- Ion conductivity developed in low-temperature-sintered bodies: Higher ionic conductivity (total: 4.0X10-4S/cm) was observed in the sintered bodies at 1,000℃.
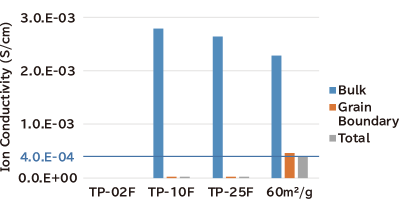
(Reference Value) Impedance-measurement by dipping LiCl aqueous solution into filter paper as electrodes
Lineup of LLTO products
- LLTO Powder (Typical Data)
- We have a variety of product lineups, including granulation, milling and nano powders.
| Item | Granulation (TP-02N) | Milling (TP-02F) | Nano powder (TP-10F) | Nano powder (TP-50F) |
|---|---|---|---|---|
| Specific Surface Area | 1.4m2/g | 2.5m2/g | 10.4m2/g | 50.3m2/g |
| Tap Density | 1.4g/cm3 | 1.5g/cm3 | ー | ー |
| SEM photograph |  | 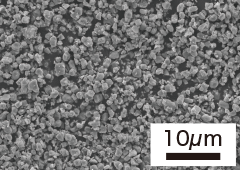 | 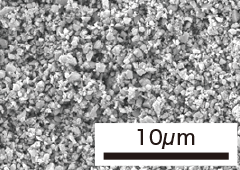 | 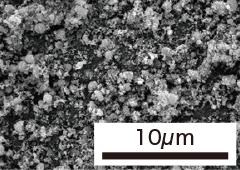 |
FAQ
What are the features of LLTO?
-
High ionic conductivity and stability in the atmosphere.
LLTO is classified as an oxide solid electrolyte.
Among such electrolytes, it exhibits a relatively high ionic conductivity (bulk) of 10-3 S/cm.
In addition, it is a stable compound and does not react with moisture in the atmosphere.
Can you provide samples?
-
Yes. Powders and sintered bodies with various specific surface areas are available, so please contact us.


